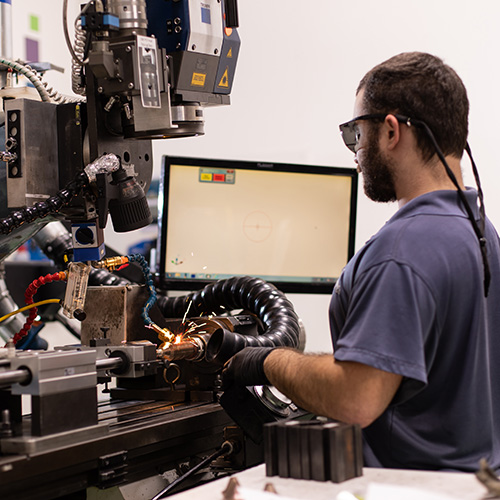
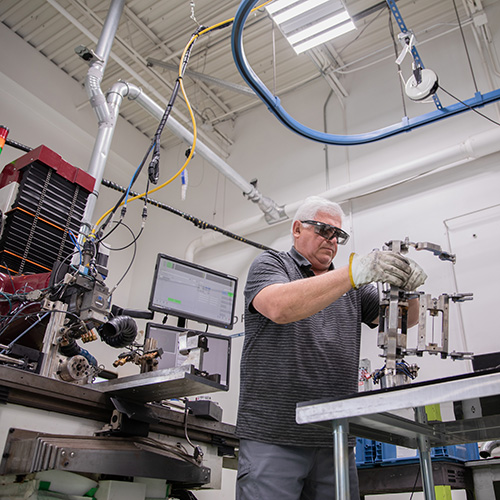
Laser Welding • Electron Beam Welding • Machining • Supply Chain Management • Resistance Welding
ENGAGE. INNOVATE. DELIVER.
OUR COMMITMENT TO YOU.
Using state-of-the-art technology and equipment, Joining Technologies develops truly innovative, high-quality welding solutions for industries nationwide — including aerospace, medical devices, automotive, energy and national defense.
When you trust your manufacturing project to Joining Technologies, your components and assemblies will be delivered on time and per specification.
Our professional teams located in East Granby, CT, USA are passionate about their work. We work in partnership with our customers to deliver successful outcomes for every project, whether it calls for precision laser welding, seamless supply chain managing or our full service machine shop.
Our specialists earn their reputations day-by-day as the most knowledgeable, efficient and professional in the industry. All along the way, we’ll guide you through the process. That means providing expert advice and selecting the best materials for the job. It means considering your customized tooling options and developing a prototype… guiding you all the way through full-scale production.
What our customers are saying:
“Joining Technologies, based in East Granby, has pioneered laser welding technology that allows us to fuse materials that have never been fused before. The resulting composites can withstand plasma-level temperatures — in excess of 11,000 degrees Fahrenheit — which are essential for producing next-generation computer chips and electric vehicle batteries.”
Boris Levin, president and
CEO of the Mott Corporation
“We have been using Joining Technologies for the decade to solve our commercial development laser welding challenges and continue to be impressed by the knowledge and professionalism of the staff and how driven they have been to work with us to solve problems.”
Velocys
“Johnson Matthey appreciates that Joining Technologies In. has successfully taken on and delivered quality service on our unique precious metals components. Our project presented JTI with challenges on many fronts, from tooling to final welding and assembly, all were met with great results! Joining Technologies is a quality and detail-oriented organization, and a pleasure to do business with!”
Johnson Matthey
“We appreciate the outstanding work fabricating groundbreaking hardware for the Viasat Program”
Boeing
“Joining Technologies lives up to their promises. Through careful planning and skillful execution in managing the supply chain for a family of products in one of our fastest-growing markets, they have supported us in every way possible, and have quickly risen to become a trusted partner in Parker’s growth strategy.”
Parker
Precision Laser Welding, Electron Beam Welding, Machining, Supply Chain Management, Resistance Welding, and more.
We are a leading North American provider of the latest in advanced laser welding, electron beam welding, supply chain management and machine shop services. We are guided by a powerful belief in teamwork and out-of-the-box thinking and we constantly strive to provide our customers with the highest quality and best possible service.